Will telemedicine remain a norm after COVID-19?

In a time of a crisis, humanity is pressured to come up with better ways of doing things. WWI brought about the use of blood banks, zippers, and the muscle strengthening technique known as pilates. ATMs, superglue, and mass-produced antibiotics were either invented or perfected during WWII.
The COVID-19 pandemic is not an exception. In the past few months, telemedical services have been exploding to the point that they struggle to onboard new customers. In companies like Amwell (a leading U.S. telehealth platform), top execs, programmers, and DevOps have been working around the clock, talking to partners, adding new servers, and expanding the network.
What is telemedicine?
Telemedicine, or telehealth, refers to giving and receiving medical help that occurs without physical contact. This may include:
- remote medical triage
- remote diagnostics
- contactless test delivery
- remote patient monitoring (e.g. of quarantined patients)
- self-help through chatbots, questionnaires, etc.
The benefits of not having to go to the clinic are obvious: it minimizes the risk of sick people infecting others, fewer doctors get sick (which is critical during an epidemic), patients get more options to choose from, and it just saves everyone’s time and effort. On the downside, it’s not always possible to get some data remotely.
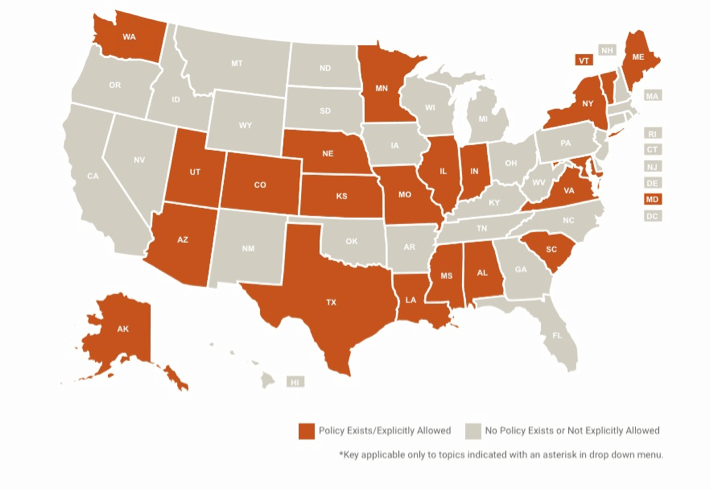
Regulatory obstacles in the way
In the United States, up until recently, few provisions were made for telemedicine in the country’s legislation. In the 1990’s, telehealth reimbursements were limited to “rural” areas where it was hard to find a doctor. While laws improved over time, they still had significant limitations.
However, amid the worsening COVID-19 crisis, the U.S. government recommended telemedicine as a “first-line defence” against the pandemic. Lobbyists and agencies moved quickly, and Congress recently passed a bill (now made into law) that gave the Department of Health and Human Services authority to waive historical telehealth restrictions.
Since then, many small tweaks were made to both federal and state regulations that relaxed the rules even further. In many states, people can now get reimbursed even for new visits (previously, they had to be so-called “established patients”) and can get reimbursed even if they have commercial insurance. Doctors can now provide services for free, which they were not allowed to do before. Plus, starting March 6, 2020, patients can get tele-help with many other diagnoses besides COVID-19.
Telemedicine during COVID-19: Concrete examples
Telemedical platforms
Amwell is a leading U.S. telemedical platform that’s been trying to help many organizations respond to the COVID-19 pandemic. According to Amwell’s Chief Medical Officer Peter Antall, the company has been busy talking to, training, and onboarding thousands of new doctors in the wake of COVID-19.
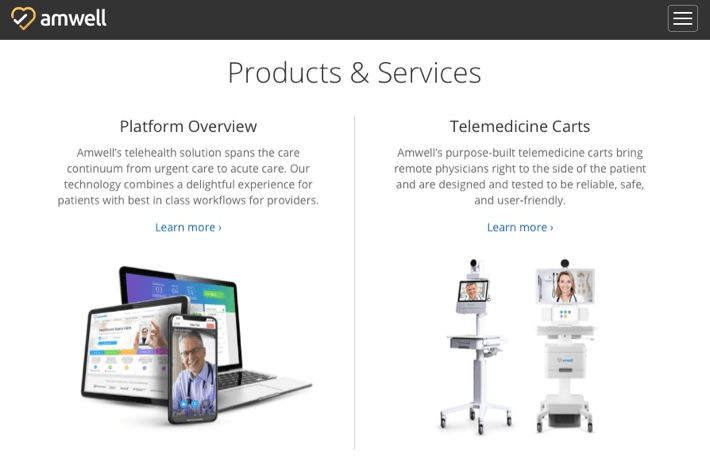
The platform essentially connects care providers to patients. It lists some 240 health systems among its clients, comprising over 2,000 hospitals and 55+ health partners with over 36,000 employees. It offers a variety of products and services, from a comprehensive telehealth platform to telemedicine carts to walk-in kiosks equipped with biometric and diagnostic devices.
Remote diagnostics
Israel’s startup TytoCare offers a range of simple use-at-home tools that allow doctors to examine patients through an app. The TytoHome Remote Exam Kit that’s available at Best Buy for $299.99 includes:
- the Tyto device (that has as a camera and a thermometer)
- a stethoscope adapter
- a otoscope adapter
- a tongue depressor adapter
It allows a physician to run a remote examination of the patient’s lungs, throat, ears, temperature, heart, abdomen, and skin. TytoCare also gives users access to a trusted physicians network through its partnership with the already mentioned Amwell telehealth giant.
In February 2020, Tyto devices were used for monitoring 12 Israelis who returned from the coronavirus-stricken Diamond Princess cruise ship. It was done to minimize the staff’s exposure to the potential infection.
Realtime remote monitoring
Another much-spoken-about company on the block is Boston-based Biofourmis. Its core offering is the Biovitals® platform – an AI-powered medical analytics system. It can spot slight physiological changes that are often precursors to actual symptoms in a patient. This helps diagnose problems early and improves patient outcomes.
In mid-February, Biofourmis was asked by the University of Hong Kong if it could repurpose its platform to algorithmically detect COVID-19 cases. The team got down to work, and three weeks later unveiled Biovitals® Sentinel, a refitted version of the original platform.

Biofourmis CEO Kuldeep Singh Rajput says that the new system collects “two million data points per patient per day” through a wearable biosensor called Everion® and through self-reported app data. This allows it to not only perfect the coronavirus-flagging algorithm, but also perform remote monitoring of patients and intervene if their condition deteriorates.
Self-help and self-diagnostics
Since COVID-19 swept the world, there appeared many grassroots tech initiatives aimed at mitigating the consequences of the crisis. But it wasn’t just medical companies or local communities that chipped in. Large techno giants are trying to play their part, too.
Apple created a COVID-19 Screening Tool that allows users to decide whether they should seek medical assistance by taking a questionnaire. The app guides you through a series of questions and prompts you whether you should seek help, stay at home and monitor your condition, or not worry at all for the time being. The app was developed in partnership with the CDC, the White House, and FEMA.
At the same time, researchers from Carnegie Mellon University decided to tackle the problem from another side. They created an AI-based experimental app that will try to predict if people have COVID-19 by “listening” to their cough and their voice.
In conclusion
As Peter Antall of Amwell put it during a telehealth panel discussion, it’s unlikely that “the toothpaste will be going back in the tube” after the pandemic. So, there is a good chance that telemedicine will simply become an integral part of healthcare in general. And while unresolved issues like reimbursement and fraud detection still remain, having working telehealth technology at hand will definitely help us weather this and any future pandemic.
Related Blogs

From robot cleaners to robo-chefs to entertainers: what kinds of robots are helping us fight COVID-19?
LEARN MORE